

Oribatid MITES (ACARI: ORIBATIDA) OF
BRITISH COLUMBIA
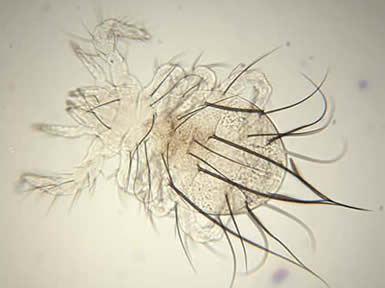
Palaeacarus hystricinus, photo by Zoe Lindo.
by
Zoë Lindo (University of Western Ontario, Department of Biology.)
and
Marilyn Clayton (Pacific Forestry Centre, Canadian Forest Service)
Checklist of Oribatid Mites of BC (2011)
Introduction
Mites (the Acari) are a highly diverse group with over 55,000 described species (Walter & Proctor, 1999). This number is vastly smaller than the true estimated number of species, which is thought to range between 500,000 and 1,000,000 species (Krantz and Walter, 2009). Mites are members of the Arthropoda having chitinous exoskeletons and jointed legs. Mites are related to spiders and separate from insects in that they lack antennae and manidibles, instead possessing chelicerate mouthparts (Chelicerata). The largest chelicerate group is the Arachnida, which along with the mites includes spiders, scorpions and pseudoscorpions, whipscorpions, and harvestmen (Shultz, 1990).
The Acari are divided into two superorders: the Acariformes and the Parasitiformes. Within the Acariformes Superorder (the mite-like mites), the Suborder Oribatida is within the Order Sarcoptiformes (Norton et al., 1993). Many changes in mite taxonomy and the phylogenetic relationship among groups within Oribatida are on-going as new species are discovered, immature forms are correlated with adults, and molecular techniques add to the information taxonomists use. The information presented here follows Krantz and Walter (2010) and the Oribatida chapter by Norton and Behan-Pelletier (2009) within which, at the time of writing, is the most up to date and reliable taxonomic and phylogenetic information available.
Suborder Oribatida – the Oribatid Mites
There are more than 9000 described species world-wide (Schatz, 2004; 2006) representing 172 families, not including the cohort Astigmatina*. Most species are litter and soil dwelling, but many are arboreal, and a few are aquatic. Temperate forests tend to have the highest densities (abundance of individuals) and species richness (100-150 species are typical). Most species are particulate feeders on decaying organic matter (saprophagous) or on the microbes associated with soil systems like fungi and bacteria (fungivous, microbivorous); but feeding also includes algivory, lichenivory, opportunistic predation and generalist scavenging, including necrophagy and coprophagy (Schneider et al., 2004). Only a few species within Oribatida are considered primarily herbivorous.
*The Astigmata were previously designated as a suborder and separate from the oribatid mites, due to significant differences in life history traits, but recent evidence places the Astigmatina within the Oribatida (Norton 1998).
Oribatid mites range in size from approximately 125 µm to 2 mm with most ranging from 300-750 µm. Oribatid mites can have low to high levels of sclerotization (or mineralization) of the cuticle, and as such tend to be white to tan, or brown to black in colour. Ontological development is via egg and retained prelarva, larva, protonymph, deuteronymph, and tritonymph to adult. Fertilization of the egg is mostly indirect via spermatophores for sexually reproducing species. The frequency for asexual reproduction (thelytokous parthenogenesis) is high and well conserved within some families (Norton and Palmer, 1991; Norton et al., 1993). Oribatid mites are relatively long-lived for their body size (estimated 1-2 years in temperate systems; 4-5 years in Boreal systems) and follow an iteroparous life history with generally low reproductive output. Oribatid mite fossils have been found from the Devonian (Norton et al., 1988), with similar species still extant. As such there tends to be a great range of derived characters, mainly associated with protection; from earlier defence strategies include erectile hairs and chemical glands (Sakata and Norton, 2001), to a range of body forms (e.g. ptychoidy (Sanders and Norton 2004)), and the development of sclerotized body parts (tecta). Predators of oribatid mites are often other mites (Suborders Prostigmata and Mesotigmata), beetles, ants, fly larva, centipedes, and small salamanders (not exhaustive).
High species richness and diversity of oribatid mites is attributed to high heterogeneity of habitat in soil systems (Giller, 1996), and microhabitat specialisation has been noted (Aoki, 1967); many species are associated with mosses (the ‘bryosphere’ sensu Lindo and Gonzalez, 2010), bark, leaf litter, needle litter, rotting wood, lichens, fresh-water, and mineral soil. A few oribatid mites are of human interest acting as agricultural and horticultural pests, agents of biological control, intermediate hosts of tapeworms, vectors of fungal disease, and ectoparasites, but the significance of this group as a pest group is very low compared to many other groups of mites and arthropods in general.
Oribatida in British Columbia
A comprehensive checklist of the oribatid mites of Canada (The Diversity of Oribatida in Canada (DOC)–available online--was compiled by Valerie M. Behan-Pelletier and Barbara Eamer of the Acarology Unit, Research Branch, Agriculture and Agri-Food Canada, Ottawa, in 2004. This built on the 1987 catalogue of the Oribatida of the United States and Canada by Marshall et al. (1987), annotated by publications and species descriptions from 1987 - 2004. The DOC checklist documents 140 species of oribatid mites for British Columbia.
The checklist compiled here includes British Columbia species records from both above-mentioned sources, with updated species names where possible. Taxonomy follows Norton and Behan-Pelletier (2009). Many new records are from the ZLC (Zoë Lindo collection) with a focus on the Coastal Temperate Rainforest (CTR) of Vancouver Island, and in particularly, canopy habitats. These species have been cross-referenced with other CTR projects such as the Carmanah Canopy Project (Neville Winchester, University of Victoria), Montane Alternative Silviculture System (Leland Humble, Pacific Forestry Centre), and Mt. Cain (Laura Fagan, University of Victoria). Other cross-referenced collections include the Royal British Columbia Museum (RBCM), with thanks to Claudia Copley and Rob Cannings, and the Canadian National Collection (CNC) of Insects, Arachnids and Nematodes, with thanks to Val Behan-Pelletier and the Acarology Unit. Also consulted were recent publications from the interior Sub-Boreal Spruce (SBS) zone by Battigelli et al. (2004), The Engleman Spruce-Sub alpine Fir (ESSF) zone by Berch et al. (2007), Montane Cordillera (Smith et al., 1998), and the work of Lindo & Stevenson (2007) from the Interior Cedar-Hemlock (ICH) forests. This species list is compiled using presence/absence data and listed by eco-zone. The final list has omitted records that include a generic identification without a specific name (e.g. Oppiella sp.) where the species is likely to be already listed (i.e. Oppiella nova); thus the list is considered a conservative estimate of the true oribatid mite diversity in British Columbia. The final count is 354 species for the province of British Columbia; of which 242 species (68%) are found within the Coastal Temperate Rainforest eco-zone (174 species (49%) are unique to the CTR in BC); though it should be noted that many areas of British Columbia are insufficiently sampled. The number of undescribed species is conservatively estimated at 25-35% of the total fauna listed here.
References
Aoki, J. 1967. Microhabitats of oribatid mites on a forest floor. Bulletin of the National Science Museum of Tokyo 10: 133–138.
Battigelli, J., J. R. Spence, D. W. Langor, and S. M. Berch. 2004. Short-term impact of forest soil compaction and organic matter removal on soil mesofauna density and oribatid mite diversity. Canadian Journal of Forest Research 34: 1136–1149.
Berch, S.M., J. P. Battigelli., and G. D. Hope. 2007. Responses of soil mesofauna communities and oribatid mite species to site preparation treatments in high-elevation cutblocks in southern British Columbia. Pedobiologica 51: 23–32.
Giller, P.S. 1996. The diversity of soil communities, the ‘poor man’s tropical rainforest’. Biodiversity and Conservation 5: 135–168.
Krantz, G., and D. E. Walter. 2009. A Manual of Acarology (3rd ed.) Texas Tech University Press, Lubbock, Texas.
Lindo, Z., and A. Gonzalez. 2010. The bryosphere: an integral and influential component of the Earth’s biosphere. Ecosystems 13: 612–627.
Lindo, Z., and S. K. Stevenson. 2007. Diversity and distribution of oribatid mites (Acari: Oribatida) associated with arboreal and terrestrial habitats in Interior Cedar-Hemlock forests, British Columbia, Canada. Northwest Science 81: 305–315.
Marshall, V.G., R. M. Reeves, and R. A. Norton. 1987. Catalogue of the Oribatida (Acari) of continental United States and Canada. Memoirs of the Entomological Society of Canada 139: 418 pp.
Norton, R.A. 1998. Morphological evidence for the evolutionary origin of Astigmata (Acari: Acariformes). Experimental and Applied Acarology 22: 559–594.
Norton, R.A., and V.M. Behan–Pelletier. 2009. Suborder Oribatida. In: Krantz, G.W. and Walter, D.E. (eds.), A Manual of Acarology (3rd ed.) Texas Tech University Press, Lubbock, Texas, pp. 430–564.
Norton, R.A., and S. C. Palmer. 1991. The distribution, mechanisms, and evolutionary significance of parthenogenesis in oribatid mites. In: Schuster R. and Murphy P.W. (eds.), The Acari: reproduction, development and life-history strategies. Chapman and Hall, London, pp. 107–136.
Norton, R.A., P. M. Bonamo, D. J. Grierson, and W. A. Shear. 1988. Oribatid mite fossils from a terrestrial Devonian deposit near Gilboa. New York. Journal of Paleontology 62: 259–269.
Norton, R.A., J. B. Kethley, D. E. Johnston., and B. M. O'Connor. 1993. Phylogenetic perspectives on genetic systems and reproductive modes of mites. In: Wrensch, D.L. and Ebbert, M.A. (eds.), Evolution and diversity of sex ratio in insects and mites. Chapman & Hall, New York. pp. 8–99.
Sakata, T., and R. A. Norton. 2001 Opisthonotal gland chemistry of early-derivative oribatid mites (Acari) and its relevance to systematic relationships of Astigmata. International Journal of Acarology 27: 281–291.
Schatz, H. 2004. Diversity and global distribution of oribatid mites - evaluation of the present state of knowledge. Phytophaga 14: 485–500.
Schatz, H. 2006. Catalogue of known oribatid mite species (Acari Oribatida) from the Central American landbridge (First part). Tropical Zoology 19: 209–288.
Schneider, K., S. Migge, R. A. Norton., S. Scheu, R. Langel, A. Reineking,, and M. Maraun. 2004. Trophic niche differentiation in soil microarthropods (Oribatida, Acari): evidence from stable isotope ratios (15N/14N). Soil Biology and Biochemistry 36: 1769–1774.
Shultz, J.W. 1990. Evolutionary morphology and phylogeny of Arachnida. Cladistics 6: 1–38.
Smith, I.M., E. E. Lindquist., and V. Behan-Pelletier. 1998. Mites (Acari). In: Smith, I.M. and G. G. E. Scudder (eds.), Assessment of species diversity in the Montane Cordillera Ecozone. Ecological Monitoring and Assessment Networks. EMAN and Partners Publications, Burlington, Ontario.
Walter, D.E., and H. C. Proctor. 1999. Mites: Ecology, Evolution and Behaviour. University of NSW Press, Sydney.
Please cite these pages as:
Author, date, page title. In: Klinkenberg, Brian. (Editor) 2021. E-Fauna BC: Electronic Atlas of the Fauna of British Columbia [www.efauna.bc.ca]. Lab for Advanced Spatial Analysis, Department of Geography, University of British Columbia, Vancouver. [Date Accessed]
© Copyright 2021 E-Fauna BC.