

The Lophophorates (Moss Animals, Lampshells and Phoronid Worms)
A brief taxonomic history
by
Aaron Baldwin, PhD Candidate
School of Fisheries and Ocean Science
University of Alaska, Fairbanks
Questions and comments can be directed to Aaron Baldwin at ftapb1@uaf.edu
Simply put, a lophophorate is any organism that bears a lophophore. This is a pair of spiral feeding structures that form a crown on the head superficially similar to the feeding tentacles of feather-duster worms. Traditionally the lophophorates include the brachiopods or lampshells, the bryozoans or moss-animals, and the phoronids as well as the entoprocts which do not have a true lophophore but are otherwise very similar to bryozoans.
The relationships between lophophorate phyla has been the source of great taxonomic confusion since they were first grouped together in the mid 19th century. Furthermore, the relationship between the lophophorates and other phyla has been heatedly debated. Morphologically, the grouping seems logical and simple enough. The lophophore is a highly derived, specialized feeding structure that is unlikely to have evolved more than once. So it would seem likely that all animals with a lophophore should be related to each other more closely than those without. Based on the presence of a lophophore and some other traits biologists in the early 20th Century erected the superphylum Lophophorata which went virtually uncontested for nearly a century (although the Entoprocta were variously included and excluded during this time).
Two other major groupings of coelomate animals (animals with a fluid-filled cavity) under the above scheme is placing arthropods, annelids, and mollusks together in one large clade (based on segmentation) and the Echinodermata and Chordates together into another large clade called the Deuterostomia, with the latter possibly including the Lophophorata. The acoelomate animals formed another large clade which included the flatworms and many other minor phyla.
With the advent of molecular taxonomies in the 1970’s and beyond, this simple picture became extremely complicated. In 1988 a paper by Field et al. based on the 18S rRNA sequence suggested that mollusks, annelids, and brachiopods group together, with the deuterostomes forming one outgroup, and the arthropods another. This highly controversial paper was contested based on a number of embryological and morphological studies, but as more and more genomic sequences were analyzed it became clear that something was wrong with the traditional classification scheme of “Lophophorata”.
For example, in repeated studies using different sequences the Bryozoa nearly always grouped as more distantly related to the other lophophorates and the mollusks. That is, mollusks and brachiopods shared a more recent common ancestor than both groups shared with bryozoans! This strongly suggested that either the lophophore evolved several times (highly unlikely) or that the mollusks evolved from a lophophorate ancestor that lost the lophophore. In 1995, Halanych et al. proposed uniting the lophophorates with the Annelida and Mollusca (as well as many other smaller phyla) into a group called the Lophotrochozoa (a combination of ‘lophophorate’ and ‘trochozoa’, the latter being annelids, mollusks, and a few other animals with a particular larval type).
In the past 15 years, the concept of the Lophotrochozoa has become firmly established and supported by many different molecular studies as well as more recent developmental and fossil discoveries. One of the more exciting from the latter has been the discovery (nearly worldwide) of an extinct group of animals called the halkeriids. Known from fragments of shell components from the Precambrian and Cambrian, the whole animal was not described until 1995. Some of the best preserved halkeriid fossils are found in the famous Burgess Shales in British Columbia. Halkeriids are a group that share features with primitive mollusks (like chitons and aplacophorans), annelid worms, and brachiopods. It is likely that halikeriids represent or are related to the ancestral forms that evolved into the mollusks and brachiopods and probably the annelids.
More recently confirmed phylogenies include the Platyzoa (platyhelmithes or flatworms, rotifers, gastrotrichs, and several other phyla) as a major clade within the Lophotrochozoa. This scheme then divides the Lophotrochozoa into three (sometimes five) broad clades including the Platyzoa, the Bryozoa, and the third group being the non-bryozoan lophophorates plus the annelids, mollusks, nemerteans, and a few others. The figure of Giribet et al. (2007) reflects a recent understanding of these groups:
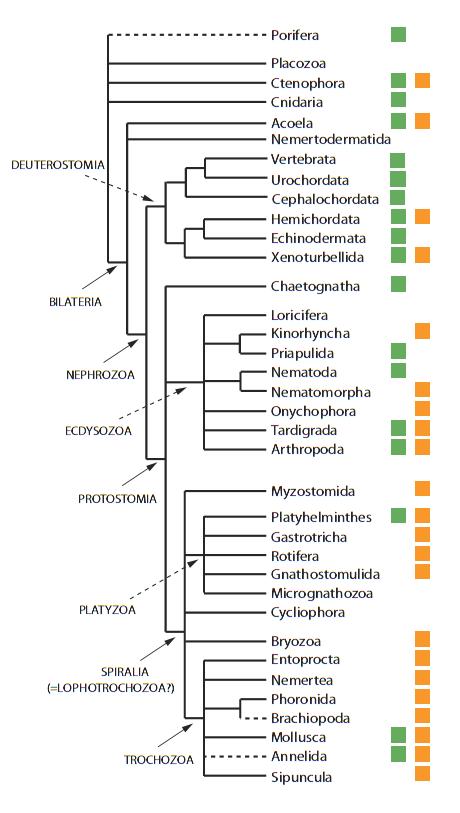
A composite cladogram showing the relationships between animal phyla. From Giribet et al. (2007).
Cladistic taxonomies aside, it is still useful to discuss the lophophorate taxa together. I will give a brief overview of each of the four lophophore-bearing phyla here.
Phylum Bryozoa:
Called the "moss-animals" this is the only large phylum of animals that are wholly colonial. Bryozoans produce a covering made of calcium carbonate, chitenous material, or mucous from which the individuals extend their lophophores for feeding. The colonies resemble and are easily confused with sessile hydroids. The common name 'moss-animals' comes from the fact that these colonies often resemble terrestrial mosses in appearance. Bryozoans are abundant in marine habitats, with about 8,000 described species. They are also abundant as fossils (over 20,000 described species!) and during the Paleozoic era formed reefs in a manner similar to modern corals. There are about two hundred species of bryozoans recorded from British Columbia.
Phylum Entoprocta:
Typically grouped with bryozoans, entoprocts differ in many ways. They lack a true lophophore (although this is debated), although they have a crown of feeding tentacles. They also have a different gut arrangement, with the anus inside the feeding tentacles (hence the name 'ento' = inside, 'proct' = anus). There are colonial as well as solitary entoprocts. This bizarre group is often found commensally on the bodies of polychaete worms, on bryozoans, and other invertebrates. I have seen an unidentified species of entoproct on the inside of the carapace of a Tanner crab shell! Some colonial entoprocts form bryozoan-like colonies in freshwater ponds in British Columbia. There are nine species of Entoprocta recorded from British Columbia.
Phylum Phoronida:
Phoronids resemble sabellid tube worms in that they are elongate and generally secrete a chitenous tube from which their lophophores extend. Unlike sabellids the body of phoronids is attached to the tube so cannot be easily extracted. This is a small phylum, about 12 species known. In most modern classification schemes phoronids are thought to be the closest living taxa to the brachiopods. There are four species of phoronid recorded from British Columbia.
Phylum Brachiopoda:
Brachiopods or Lamp shells are a group that is best known from fossils. They superficially resemble bivalved mollusks, but the well-developed lophophore and peduncle quickly separate them from that group. Brachiopods are common in some habitats, but there are only about 300 species alive today. This is amazing considering there are about 5,000 species known from the Paleozoic. In both species and higher level taxonomy the Permian extinction event devastated the diversity of this group. Several brachiopod species are common in the lower intertidal and subtidal areas of British Columbia. There are seven species of Brachiopoda recorded from British Columbia
References
Bernard FR (1972) The living Brachiopoda of British Columbia. Syesis 5: 73-82
Boardman RS, Cheetham AH, and Rowell AJ (1987) Fossil Invertebrates. Blackwell Science. 728 pp.
Brusca RC & Brusca GC (2003) Invertebrates, 2nd edition. Sinauer Associates. 936 pp.
Field KG, Olsen GJ, Lane DJ, Giovanni SJ, Ghiselin MT, Raff EC, Pace NR, and Raff RA (1988) Molecular phylogeny of the animal kingdom. Science 239: 748-753.
Giribet G, Dunn CW, Edgecumbe GD, & Rouse GW (2007) A modern look at the animal tree of life. Zootaxa 1668: 61-79.
Halanych KM, Bacheller JD, Aguinaldo AMA, Liva SM, Hillis DM, & Lake JA (1995) Evidence from 18S Ribosomal DNA that the lophophorates are protostome animals. Science 267: 1641-1643.
Helmkampf M, Bruchhaus I, and Hausdorf B (2008) Multigene analysis of lophophorate and chaetognath phylogenetic relationships. Molecular Phylogenetics and Evolution 46: 206-214.
Hochberg FG (1996) Brachiopoda. p. 1-74. In: Taxonomic Atlas of the Benthic Fauna of the Santa Maria Basin and Western Santa Barbara Channel. Santa Barbara Museum of Natural History, vol. 14, Miscellaneous Taxa, 305 pp.
Kozloff, E. N. 1996. Marine Invertebrates of the Pacific Northwest with additions and corrections. University of Washington Press, Seattle WA. 539 pp.
Lamb A and P. H. Hanby. 2005. Marine Life of the Pacific Northwest. Harbor Publishing, Madeira Park, BC. 398 pp.
Marsden JCR (1959) Phoronidea from the Pacific coast of North America. Canadian Journal of Zoology 37: 87-111.
Massard, JA and Geimer, G (2008) Global diversity of bryozoans (Bryozoa or Ectoprocta) in freshwater. Hydrobiologia 595(1): 93-99.
Odonoghue CH & O’Donoghue (1923) A preliminary list of Polyzoa (Bryozoa) from the Vancouver Island Region. Contributions to Canadian Biology and Fisheries 1:143-201.
Odonoghue CH & O’Donoghue (1926) A second list of the Bryozoa (Polyzoa) from the Vancouver Island Region. Contributions to Canadian Biology and Fi9-131.
Okamura B & Wood TS (2002) Bryozoa as hosts for Tetracapsula bryosamonae, the PKX organism. Journal of Fish Diseases 25: 469-475.
C (1950) Bryozoa of the Pacific coast of America. Part 1, Cheilostomata – Anasca. Allan Hancock Pacific Expedition 14(1): 1-269. Available at: www.archive.org/stream/bryozoaofpacific01osbu/bryozoaofpacific01osbu_djvu.txt
Osburn RC (1952) Bryozoa of the Pacific coast of America. Part 2. Cheilostomata – Ascophora. Allan Hancock Pacific Expedition 14(2): 271-611. Available at: www.archive.org/stream/bryozoaofpacific02osbu/bryozoaofpacific02osbu_djvu.txt
Osburn RC (1953) Bryozoa of the Pacific coast of America. Part 3, Cyclostomata, Ctenostomata, Entoprocta, and addenda. Allan Hancock Pacific Expedition 14(3): 613-841. Available at:
www.archive.org/stream/bryozoaofpacific03osbu/bryozoaofpacific03osbu_djvu.txt
Petersen KJ and Eernisse DJ (2001) Animal phylogeny and the ancestry of bilaterians: inferences from morphology and 18S rDNA gene sequences. Evolution and Development 3(3): 170-205.
Reynolds JD (1976) Occurrence of the freshwater Bryozoan, Cristatella mucedo Cuvier, in British Columbia. Syesis 9: 365-366.
SCAMIT (2001) A taxonomic listing of soft bottom macro- and megainvertebrates from infaunal and epibenthis monitoring programs in the Southern California Bight, Edition 4. Southern California Association of Marine Invertebrate Taxonomists. www.scamit.org/edition4/Edition%204B.pdf
The Bryozoa Home Page http://www.bryozoa.net/index.html
Please cite these pages as:
Author, date, page title. In: Klinkenberg, Brian. (Editor) 2021. E-Fauna BC: Electronic Atlas of the Fauna of British Columbia [www.efauna.bc.ca]. Lab for Advanced Spatial Analysis, Department of Geography, University of British Columbia, Vancouver. [Date Accessed]
© Copyright 2021 E-Fauna BC.